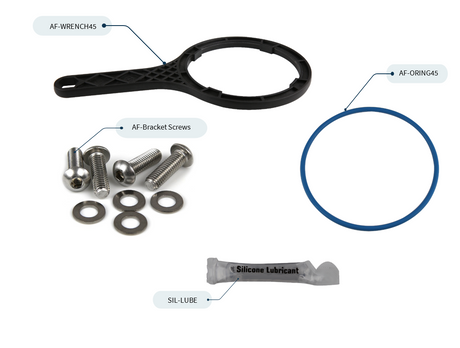
Replaced old salt water treatment system with the Cascadian ICS-TP system in 2017, and we're extremely satisfied!
The complete system takes up less than a third of the space of the old system, it's much easier to replace the filters than making a mess with the rock salt, their video providing step by step directions of replacing filters is outstanding, the staff is experienced and helpful, and the water is cleaner and softer resulting in less hardwater buildup of our faucets.
With what we've experienced, we'd choose the Cascadian ICS-TP system 100 times out of 100 over ANY other system!